Search Results
Results for: 'partial pressure'
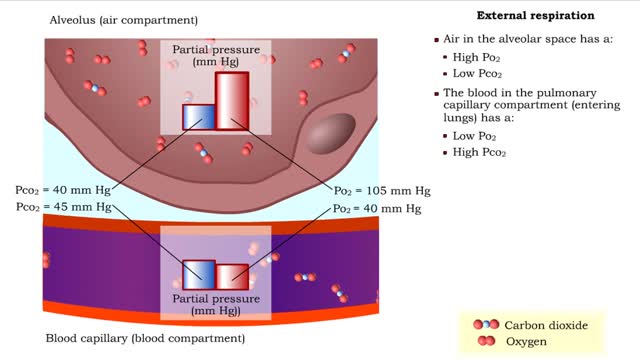
Gas exchange - partial pressure, locations, external and internal respiration
By: HWC, Views: 6808
▪ In a mixture, each individual gas exerts a pressure that is proportional to the concentration of that gas within the mixture. • This part of the total pressure is called a "partial pressure". • A gas moves along the part of the pressure gradient determined by its own concentration. ...
Double Vacuum Process (Illustration No Audio)
By: HWC, Views: 5881
In this so-called low-pressure process, the wood is first subjected to a short and relatively weak initial vacuum, after which the treatment vessel is flooded with preservative solution and reduced to normal pressure The double vacuum container is loaded with timber. A partial vacuum is draw...
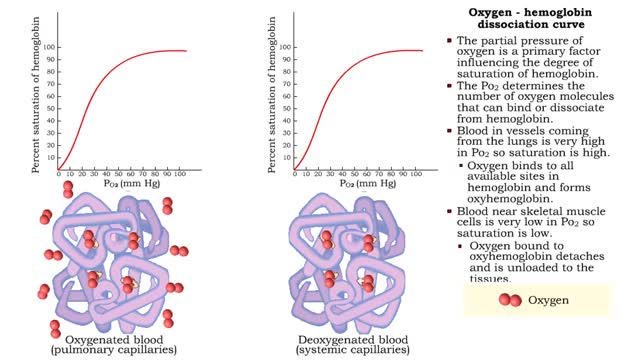
Oxygen - hemoglobin dissociation curve & Hemoglobin's affinity with oxygen - acidity
By: HWC, Views: 7290
• The partial pressure of oxygen is a primary factor influencing the degree of saturation of hemoglobin. • The Po2 determines the number of oxygen molecules that can bind or dissociate from hemoglobin. • Blood in vessels coming from the lungs is very high in Po2 so saturation is high. ...
Hydrogen bonds - role in the body
By: HWC, Views: 7011
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
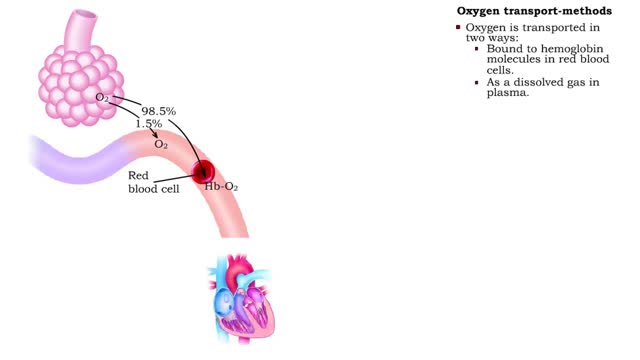
Oxygen transport - methods and oxyhemoglobin
By: HWC, Views: 6418
• The blood is the medium used for gas transport throughout the body. • Oxygen is only available in the lungs. Because the partial pressure of oxygen is higher in the alveoli than in the blood, oxygen diffuses into the blood and is transported to systemic cells. • At the tissues the par...
By: Administrator, Views: 9606
How nurses check a patient's blood pressure. Blood Pressure The pressure exerted by the blood on the walls of the arteries. Higher (systolic) number: the pressure while the heart contracts. Lower (diastolic) number: the pressure when the heart relaxes between beats. Measured by a sphygmoma...
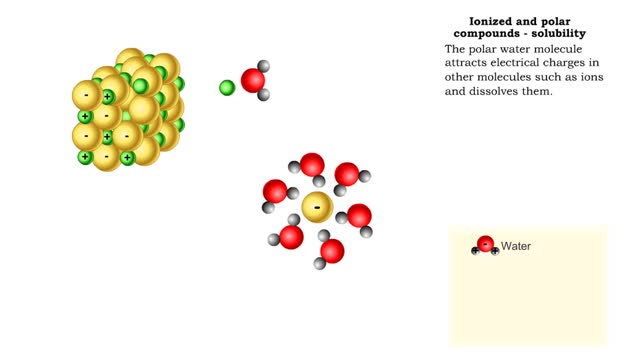
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 6823
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
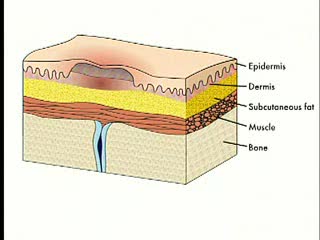
By: Administrator, Views: 9736
Pressure ulcers, also known as bedsores, decubiti, decubitous ulcers, pressure injuries, and pressure sores, are localized damage to the skin and/or underlying tissue that usually occur over a bony prominence as a result of usually long-term pressure, or pressure in combination with shear or fric...
Non-polar compounds - insolubility
By: HWC, Views: 6688
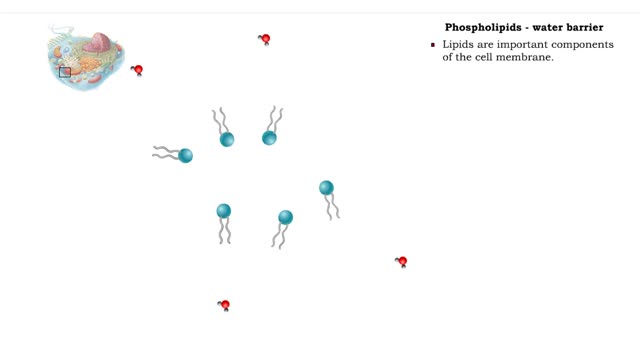
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
Advertisement